Develop a reliable and innovative medical device
From concept to production, Altyor guides you in bringing your medtech hardware innovations to life.
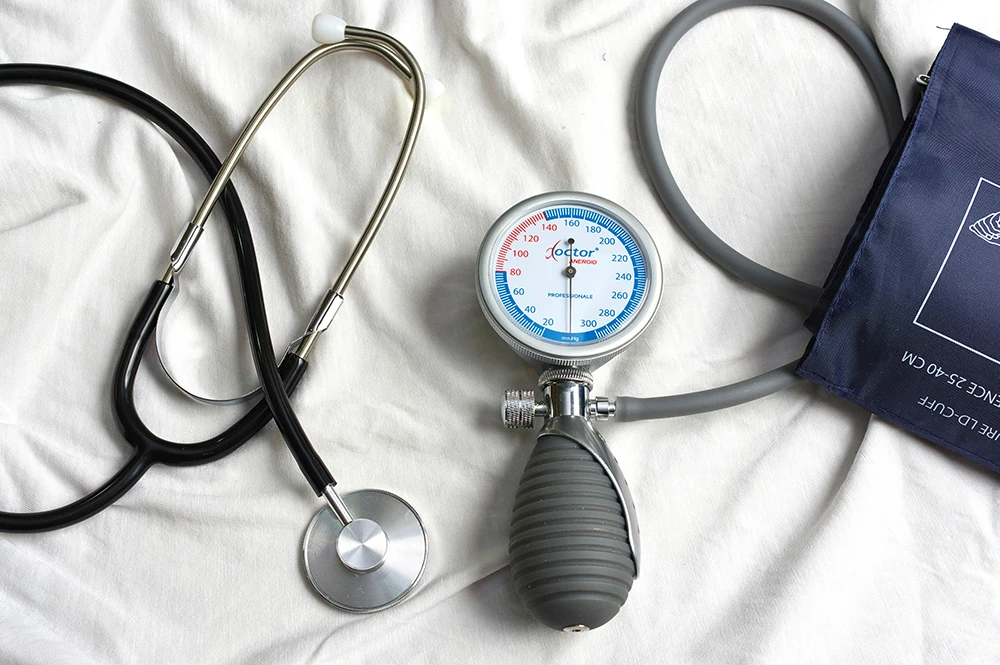
The challenges of developing
medtech electronic products
Used for diagnosis, treatment, monitoring, or improvement of care,
medical devices must meet major technological and regulatory challenges.
From ideation to manufacturing your product
From concept to certified product, we control 100% of the value chain.

Industrial manufacturer for
over 25 years

+ More than 700,000 products/year
including 150,000 in France

Responsible manufacturing
Hardware products
launched on the Medtech market

CBA informatique
Health insurance card reader
Bloomy is a insurance card reader designed for self-employed professionals on the go. Equipped with Bluetooth (BLE) and USB-C connectivity, it combines robustness and autonomy to adapt to the constraints of mobile professions, while ensuring efficient and reliable use.
Altyor’s role: Design, industrialization, production

Life AZ
Connected defibrillator
Clark is a portable connected defibrillator that does not need to be plugged in. It also provides step-by-step voice and visual guidance. With a battery life of up to 5 years, it guarantees enhanced reliability.
Altyor’s role: Design, mechanical engineering, industrialization, and production of the casing

Sevenhugs
Sleep tracking system
HugOne consists of contactless sensors that record total sleep time, sleep phases, and heart rate and breathing measurements. There is no need to wear a wristband; the sensors are placed under the mattress. The associated phone app receives and analyzes the information.
Altyor’s role: Design, industrialization, and production
Your project deserves a reliable industrial partner
Let’s discuss your needs and evaluate the best options together.
FAQ – Development and industrialization of medtech products
How long does it take to develop a medical hardware device?
The development of a medical hardware device depends on its risk class, technical complexity, and regulatory requirements.
On average, a project is structured as follows:
Product design & architecture: 4 to 12 weeks
Functional prototyping: 8 to 16 weeks
Technical testing and iterations: 8 to 20 weeks
Preparation for industrialization: 12 to 24 weeks
At Altyor, our experience in critical products and regulated environments allows us to secure each stage and optimize time to market, without compromising on quality or compliance.
What are the costs of developing a medtech hardware product?
The budget for a medtech hardware project depends in particular on:
– The level of safety required
– Reliability and traceability constraints
– The certification process
– Production volumes
Altyor’s approach consists of optimizing technical and industrial choices from the outset in order to control costs throughout the product lifecycle.
Is it possible to produce in small batches?
Yes. Small-batch production is a common step in medtech projects.
Altyor enables you to:
– Produce industrial pre-series
– Secure the first production batches
– Test the product in real-world conditions before ramping up production
This step-by-step approach reduces technical, industrial, and regulatory risks.